July 2017
Sorting through seal thoughts
MacKenna Newmarch, undergraduate student
1 July 2017
Hello!
My name is MacKenna Newmarch - I am a fourth-year marine biology student and I will be taking over the Whatcom Creek project this summer and coming school year. Studying marine mammals in the Pacific Northwest has been a dream of mine since a young age and it is overwhelmingly thrilling to see these dreams blossom as I take part in projects like these. I have been with this lab group for over a year and cannot wait to put my own spin on the data we are collecting from our whiskered friends with the help of my trusty assistants.
While the project leads previously have asked extremely interesting questions about how many individual seals are in the creek, how many salmon they are eating, what time of day they visit the creek and the state of the tide, I am now interested in the possibility of tying these data together into one question: how do individuals vary in the times and tides they visit the creek and when are they eating the most salmon?
Since we recently have been able to identify individuals (more than 120 from 2011-2015) from photos, we can use this information to keep track of the time of year and day those individuals come into the creek, behaviors each of them are displaying and how often, and when they are eating. This information can add to information we already know about how Harbor Seals feed on salmon, but on an individual level we could inquire how much this varies.
I have considered looking into the repetitive behavior of seals as well, using thes same data. While working in the lab, I noticed coincidentally that the same seal showed up at exactly the same hour and minute the next day at the exact same location of the creek. This raises the question: are seals purposefully practicing the same behaviors at the same time of day? How are they able to time this so precisely and using what senses? Why is this beneficial to them?
Lastly, I have been interested in regularly taking water quality samples in the creek, possibly every two weeks, testing for pollutants from runoff to see if this affects the number of fish we see seals catching, fishermen catching, or simply overall reports of salmon returns from the hatchery. It would be especially compelling to test for copper, which is found in most vehicle brake pads. Copper is freed from the pads upon friction with the wheel and can be washed into waterways from streets when it rains. This heavy metal is known to inhibit salmon’s olfactory system which can prevent them from making it back to their home streams to spawn which would theoretically result in less salmon being caught by seals. Since this would require water quality data from several different years to provide anything meaningful, it’s possible that we could find organizations that have already been collecting this information nearby for many years.
I am quickly finding that there are many questions I have about our seals despite the plethora of information we have gathered about them through the years. It will be difficult to narrow this down and focus on one single question, but I am hoping that as I continue to read through recent and past studies on Harbor Seals that I will find what is most interesting to me and would benefit the scientific community and public the greatest. As Lawrence Krauss said in one of my favorite podcasts, Waking Up with Sam Harris, “science is not a set of facts, it’s a process for discovering facts.” I fully intend to honor that definition by contributing to the world of science in discovering at least one new fact about Harbor Seals. If I can accomplish this goal, I will be nothing short of ecstatic.
References
- Baldwin, D.H., Sandahl, J.F., Labenia, J.S., Scholz, N.L. (2003) Sublethal effects of copper on coho salmon: Impacts on nonoverlapping receptor pathways in the peripheral olfactory nervous system. Environmental Toxicology and Chemistry 22:2266-2274. DOI: 10.1897/02-428.
- Harris, S. (2017, 10 April) Waking up with Sam Harris #70 Beauty and Terror. Audio blog post. https://soundcloud.com/samharrisorg/70-beauty-and-terror
A new perspective on marine mammal research
Raven Benko, undergraduate student
1 July 2017
This last month I have been working with our graduate student, Madelyn Voelker, and one of her Principal Investigators, Dr. Dietmar Schwarz, on research for her graduate thesis and I have been learning a lot of new skills for studying marine mammals in the process. Madelyn is working with harbor seal DNA extracted from scat that was collected from a variety of sites in the San Juan Island area. I was actually a part of a few of the collections and it was very fun to drag an SUV full of gear into our research vessel - a small four person motorboat - travel out to our collection sites amidst harbor porpoises, harbor seals, and the occasional sea lion and elephant seal, and collect scat samples from various haul-out structures. We always ended our days pleasantly exhausted.
The work that I am doing this summer is perhaps less glamorous but equally as interesting. We are working to process all of the scat samples that were collected last summer and several steps must be completed to begin answering the kinds of questions Madelyn is interested in. She is doing a diet analysis on harbor seals in the area in an attempt to explore the feeding preferences of different sexes and even different individuals. Her work will give us insight into how harbor seals are affecting threatened fish populations such as Chinook salmon. I am currently helping Madelyn with the individual identification portion of the project in which we are amplifying various microsatellites in each DNA sample to differentiate samples as individuals. Microsatellite are small, highly repetitive segments of noncoding DNA that, due to their repetitive nature, have high mutation rates. Microsatellites are often used as markers to identify individuals because two individuals very rarely have the same combination of these markers.
Currently, my job is to dilute our DNA samples to the standard 20ng/μL if needed (it turns out extracting DNA from seal scat is not extremely efficient and many of our samples have DNA concentrations below 20ng/μL); set up and run Polymerase Chain Reactions (PCRs) using specific primers that amplify a single microsatellite; make an agarose gel and run the PCR products through the gel using a technique called gel electrophoresis; and finally, image the gels to determine if our technique has worked and which samples contain the microsatellite in question. It has all been extremely interesting work and I have been able to work on my molecular bench skills including proper pipetting techniques, sterilization protocol, and general lab notebook upkeep.
I have certainly been busy this summer, and my to do list is only growing as I start to get prepared for my first oral presentation at a scientific conference in July: the Larval Fish chapter at the Joint Meeting of Ichthyologists and Herpetologists in Austin, TX. More to come on both the larval fish and harbor seal fronts in July!
Samples versus sun
Madelyn Voelker, graduate student
6 July 2017
Summer is officially in full swing in Bellingham. The weather is consistently beautiful, the amazing mountain hiking trails are quickly melting out, and the water (in the lakes) is getting warm enough to swim in. However, my research is in full swing as well! Both of these things are GREAT but having them hit at the same time has created an internal struggle of sorts. Now that I am a couple weeks into the summer-research wars, I have had time to think about the dynamic.
One of the best things about being a graduate student doing research is making your own schedule. Additionally, I am lucky enough to have advisors who trust me to be productive without someone looking over my shoulder. This means that I make most of my own productivity goals, and am responsible for holding myself to them. As I alluded to above, this is a blessing and a curse as it requires me to police myself, and my work ethic, in a way that I have never had to before. This is a very valuable experience but leaves only me standing between myself and the sunny outdoors (queue the internal struggle). Thus, I have started looking for ways to better manage the situation to make sure I am not falling behind in my research.
One of the cheesier tools I have come up with is a fill-in-the-progress-thermometer, like you would see at a fund-raising event. I did the math to figure out how many samples need to be processed for hard-parts each week to get them all done by a date at the end of the summer. Every week I draw up a new thermometer and fill it in as we go. We get to see our progress (feels good!) and it acts as a reality check when I get distracted or tempted by the sun.
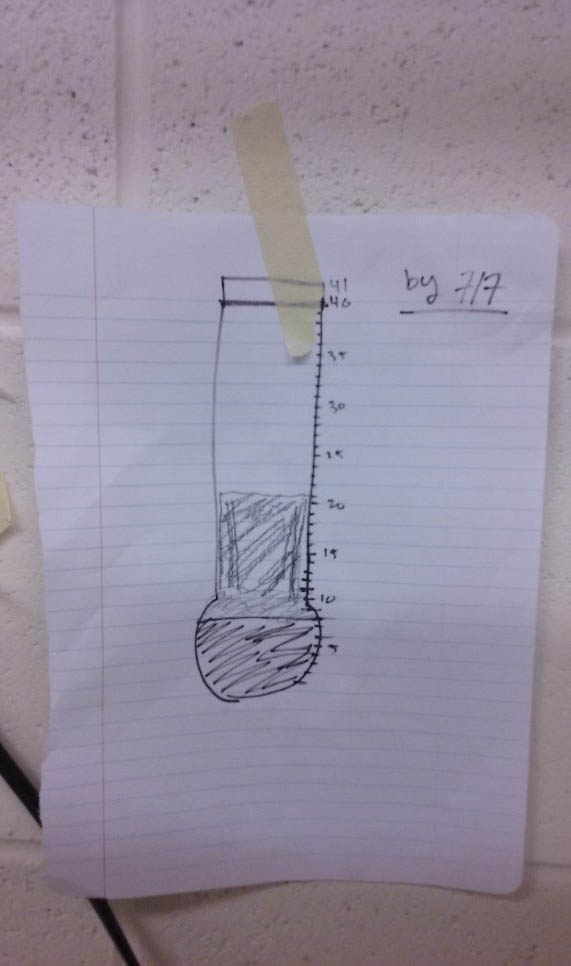
Here is this week's. Half way there - not bad!
Also, the thermometer definitely does not have to be pretty to work.
A second tool I have been applying is a simple trick I used to use when studying during finals week. This is planning out exactly how long you want to on each task every day. This gets tedious sometimes, but it keeps me working for the entire time I have planned out. Additionally, it helps me see what is taking longer than expected and makes it obvious if I am not getting done everything that I expected too.
I am by no means a master at self-motivation, nor do I have a perfect work ethic. However, this summer is allowing me to really work on these skills – for me, graduate school has been about much more than learning how to conduct science. All I can say is I am grateful I am choosing between two things I really enjoy! So, enjoy the official start of summer!! (For those of you not from here, the definition of the first day of summer in the Pacific Northwest is actually not the summer solstice, it’s the beginning of July)