May 2019
Methods papers
Alisa Aist, undergraduate student
1 May 2019
As I am wrapping up my time at Western I am writing a methods paper about how I conducted my surveys. It’s kind if funny that I am doing research on how to write up my research, but methods papers are quite a bit different than the research papers I have always written for classes. In a method paper the design of the experiment is the focus with an experiment being an example of how the methods work. The goal of the methods paper is to have the reader feel like to could exactly replicate what you did and they will know why you did each part the way you did.
There are lots of way to write methods papers, but one example followed the typical introduction/background, methods, results, discussion but with more subheadings. You still have a basic introduction, but then once you get into the methods section you end up going really in depth. It is important to explain how the participants were selected, especially in the type of project that I doing. When it comes to surveys getting a representative and random sample is one of the hardest things you have to do. That is probably the part of the project that took me the longest to get through. I had to pick a method of picking houses that took into account that neighborhoods can be made of sections that are more homogenous than the neighborhood as a whole. There is also the problem that most places where we knock on doors will not open or want to participate. So you have to knock on many more doors than participants you need. After participants you need to outline your materials. This includes what you use to share your survey and the program the survey is in. Then it’s the actual procedure which puts both of the other sections into practice. This should cover in depth the execution of the experiment, or in my case the survey. It will be this section that will really allow the reader to truly duplicate your methods. The results section also focuses on methods, but now those methods are the statistical analyses you run. Each result needs to be explained in terms of how it was found. Then there is the discussion, it starts much the same as in other papers, but when you think about the implication of your work you focus on what issues your methods solved, not what your experiment necessarily found. The benefits of the methods could be directly related to what you can then say about the results of the experiment.
I don’t think I will be following this layout of a methods paper exactly, but it was great to read what the professor thought a methods paper should be. Methods papers seem to be pretty flexible in format, but they also tend to be much longer and more specific than other research papers. Because they are so long though they do all tend to contain a lot of the same information if not in the same order.
Rizopoulos and Leek (2016).USC: Organizing Social Sciences Research Paper.
Kaiser: Research Methods Paper.
April 2019
Nathan Guilford, graduate student
1 May 2019
This month I have been able to make headway in the sampling scheme for my project, with some advancements in sample sourcing that I am very excited about. One development in my sampling is the sourcing of captive scat samples from one known seal at the Seattle aquarium, which will serve as a control in my genotyping-by-sequencing method. I have also acquired a set of seal rectal swabs from my committee member, Austen Thomas, that were taken with WDFW back in 2015. These samples will be sequenced alongside the scat samples, as we are expecting these swabs will need to be enriched as well due to an excess of bacterial DNA.
We have also decided to expand my project into two separate sequencing runs, one of non-invasive samples and one of tissue samples. As I have a new sample type (rectal swabs) that need to be enriched alongside the scat samples, we did not want to overload one sequencing run with the non-invasive samples and tissue samples, resulting in a low sequencing coverage. Therefore, the first run will sequence my collection of non-invasive samples: 8 individual wild scats, 3 captive scats from one individual, and 4 individual wild rectal swabs. The wild scats and rectal swabs will be sourced from an even distribution from Comox, BC to Whidbey Basin, WA in order to obtain a robust, informative panel of SNPs for future analyses in the Salish Sea. Four of the wild scats are thought to be resamples from 2 separate individuals based on our lab’s previous work, and I will sequence these in order to test this finding. The rectal swabs will serve as a semi-invasive sample type, the results of which I can compare to the scat samples. Should the swabs perform significantly better, this sample type would still be proven as a novel, less-invasive tool for individual specialization investigations, as prey DNA should still be present in the swabs and preserved through enrichment. The 3 captive scat samples will come from one known male at the Seattle Aquarium, and will act as a control for my genotyping process, as I should see 3 samples return with matching genotypes.
In order to evaluate the success of the fecal enrichment prior to sequencing, I will perform a second sequencing run on 5, high-quality tissue samples from stranded seal carcasses. This pure, concentrated DNA will provide reads solely from the seal genome, allowing me to compare the reads from the first sequencing run and evaluate if the non-invasive enriched DNA is adequate for marker identification/genotyping. The reads from this run will also add valuable genetic diversity to the pool of seal reads from the first sequencing run, allowing me to identify the most informative SNPs for the final panel.
Clearly, with this extension of my project comes an extension to my budget, and I am now working on sourcing the funds for this second sequencing run. Luckily, due to the low-cost of sequencing these days, the added cost is not exorbitant, and I feel hopeful about locating the funds necessary through various sources of support I am currently applying for. Once these funds have been secured, and I have chosen supplementary scat samples to those mentioned above from the vast collection in our freezers, I should be able to have that first sequencing run performed in early summer. Following procurement of tissues from the stranding network in July, I will then have the second run performed in late summer. This staggering of the sequencing runs should help me deal with processing the high volume of data I will receive, as well as allowing me to draw some conclusions about the performance of the scat sequencing by the time I receive the tissue sequence data.
This quarter has been steadily picking up in speed, and May seems to be following that trend with several presentations. However, I am excited for the chance to present my proposed project as a poster at both the WWU Graduate Symposium and the 2019 meeting of the Northwest Student Society for Marine Mammalogy. This should give me great experience in talking about my project, and a chance to bounce ideas off of other marine mammal ecologists. One thing that definitely helps is the return of sunny weather, and I’m getting excited about spending my first summer in Bellingham!
Presentation
Wyatt Heimbichner Goebel, undergraduate student
1 May 2019
April has been a whirlwind. On top of my regular commitments, I had the opportunity to compete as part of the WWU Slam Poetry Team at the College Union’s Poetry Slam Invitational (CUPSI) in Houston, Texas. In addition, I was able to attend, and present at, the Pacific Northwest Writing Center Association conference in Yakima, WA. Unfortunately, these wonderful experiences were paired with my car breaking down during the first week of April. My car breaking down threw a wrench in my plans as it significantly hindered my ability to travel to Semiahmoo Marina to do observations for my project. However, Alejandro and I came up with a plan for presenting my project as it currently stands even if I won’t be able to collect the data that I was planning to collect. We are fast approaching Scholar’s Week here at WWU and the Northwest Student Society for Marine Mammalogy Conference, which both take place during the middle of May. As such, my efforts for the past couple weeks have focused on fine tuning my methodology and working on a poster that I will be presenting at Scholar’s Week and the Marine Mammal Conference.
The plan for presenting my research at this point is to present it as a proposed study. Proposed research is totally acceptable at both Scholar’s Week and the conference. Creating a poster about a proposed study is a little bit different than creating a poster for research that has already been completed. It will start out the same as it will have an introduction and methods section. However, instead of having results and discussion, it will have a section for expected results and future directions. I plan to go in-depth on my methodology as that is one of the most important parts of a proposed study. I also plan to add graphs and a table that represent my hypotheses in the expected results section. The future plans section will essentially discuss how data collection will proceed into the future. To prepare to make the poster, I have been going back through papers I’ve read, making sure my methods are solid, and getting an idea of the number of seals and noise level present at each site. I have about a week and a half left and this should be plenty of time to complete my poster.
April 2019
Jonathan Blubaugh, graduate student
1 May 2019
The weather is improving, and the new quarter is going well. Teaching has steadily gotten easier as I get more familiar with the material and more confidence in being in front of the class. It is very nice to have the sun up when I get to school and it still be up when I leave, a big difference from winter quarter! I have also gotten my thesis proposal approved though I still have few edits to work on before I can call it the beginning of my thesis. My project has also changed a bit in scope over the past couple weeks. I had a meeting last week with Isaac Kaplan from NOAA to talk about available data to parameterize my model. He said that it would be easier to parameterize the model if I increased my study area to include the Puget Sound. The amount of work to model just the Strait of Georgia is not significantly less than to include the Puget Sound also so my committee and I have decided to include the larger area. This is also beneficial because if I want to do any time series fitting, the only time series data available is for the Puget Sound study area. I have included a figure that shows the new study area from my project. I expect to get the data to begin parameterizing the model in the next week or two and I am extremely excited.
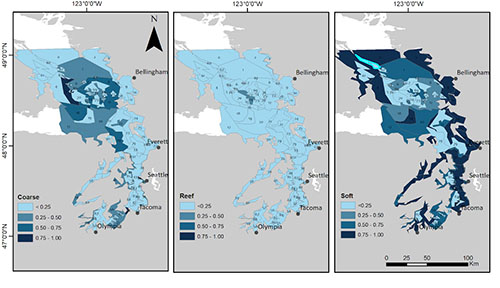
Puget Sound: the new study site.
Data analysis, poster prep
Madison McKay, undergraduate student
2 May 2019
I cannot believe it is already May! Time really does fly. I am happy that the sun is finally coming out, and I am starting to feel excited to graduate instead of being overwhelmed by everything there is to do. It will all be over soon! Looking back over the past month I am proud of everything I have accomplished. I finished my abstract which was accepted for both scholars week and the marine mammals conference, I finished most of my data analysis, and I will be finishing my poster within the next week. Not to mention we have been interviewing new lab members which will be an exciting end to spring quarter.
Data analysis
With factors including the number of fishermen, number of seals,
number of salmon arrivals, number of fish caught by seals
(response) and year (random factor), I used a linear model to
determine which factors best predict hunting success in seals.
When I first ran the model, I found that the best predictors were
the number of seals as well as the number of fishermen present.
However, I thought that there might be some gaps in the salmon
arrival statistics so I refined my data set and ran it again. This
time, I only included data points where the salmon arrivals were
over 100. With this new data set, I found the best predictor for
hunting success was only the number of seals present.
Because it is hard to tell if the salmon arrival numbers are telling of the actual number of salmon in the creek, I chose to focus on the second data set which only includes high arrivals. From this data set I can conclude that sport fishermen are not affecting the hunting success of the seals, and the best indicator for success is the number of seals present. I am now interested in investigating whether the seals are working together to increase hunting success, or if the number of seals is simply a better indicator of the number of salmon present.
To do this, I am going through old photos of seals and trying to measure the distance between individuals. This is difficult because even if they are close together, it is hard to tell if they are working together or competing for salmon. I think that if I combine these distances with more anecdotal evidence from our observations I will have a good start.
Poster
I have my poster outlined, and will be done within the next week.
I am unsure if I will have any conclusive data on the interactions
between seals, but I will at least be able to provide some
evidence and elude to further exploring the idea. I am very
excited about this because one of my first ideas for my seal
project was to look at the interactions between seals. It is all
looping back to the beginning!
What an exciting time. I can’t wait to present, hire new lab members, and pass on the ropes to Delaney.